
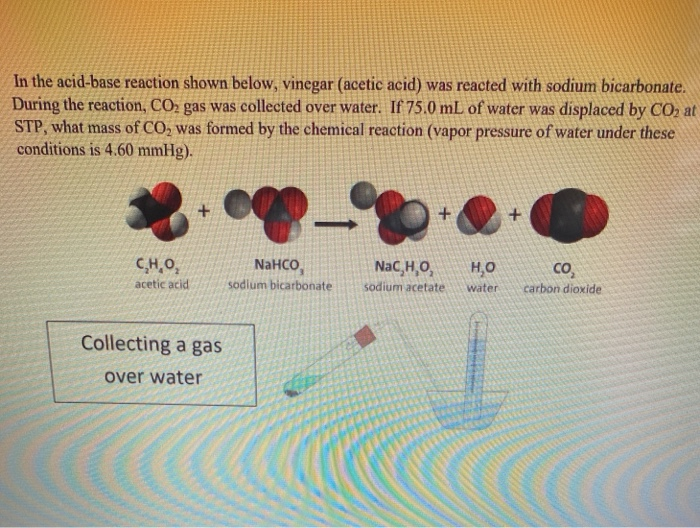
SOLVED: Sodium acetate (NaC2H3O2) is a basic salt. When sodium acetate is dissolved in water, it dissociates into its component ions. This reaction goes to completion, as indicated by the one-way arrow

SCH 4 U 1. What are buffers? Buffers are mixtures of conjugate acid- base pairs that allow a solution to resist changes in pH when acids and/or bases. - ppt download

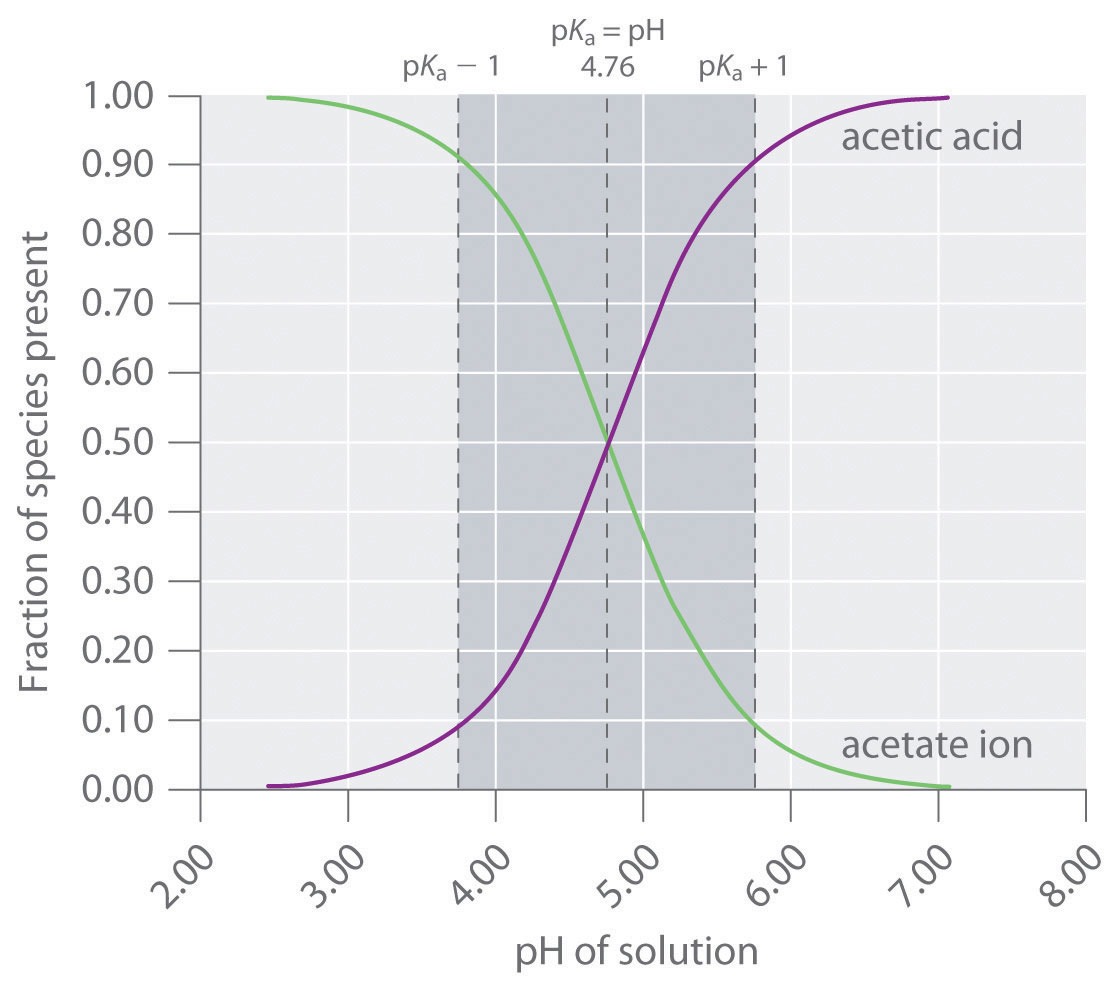
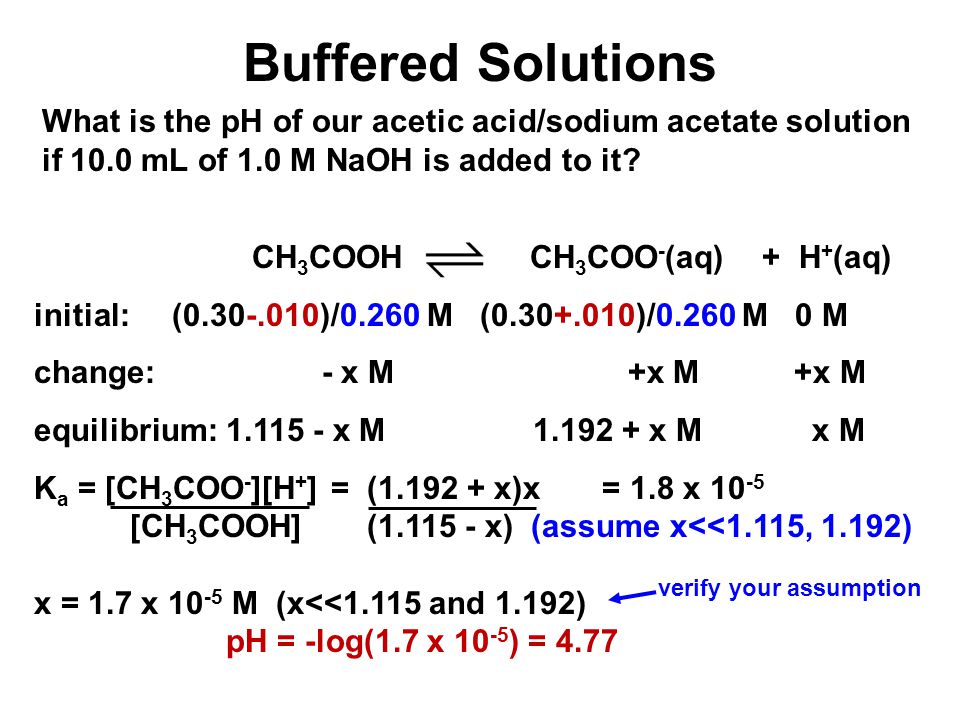
1 Chapter 10 Acids and Bases 10.9 Buffers. 2 When an acid or base is added to water, the pH changes drastically. A buffer solution resists a change in. - ppt download

Sodium Acetate(CH3COONa) - Structure, Properties, Preparations, Uses, Important questions, FAQs of sodium acetate.

You have 250mL of a 0.56M solution of sodium acetate. How many mL of 0.50M acetic acid should be added to make a buffer of pH 4.40? | Homework.Study.com

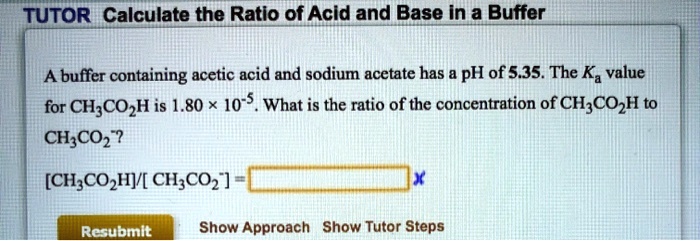
50 mL of 0.1 M solution of sodium acetate and 50 mL of 0.01 M acetic acid are mixed. The pKa of acetic acid is 4.76. The pH of the buffer solution is:

What is the pH of buffer solution containing 0.17 M acetic acid and 0.36 M sodium acetate? - YouTube
Why does the solution of sodium acetate give more concentration of Hydroxide ion? Shouldn't the number of Hydroxide ion and hydrogen ion be equal? - Quora

If sodium acetate is a weak acid and does not readily dissociate in water or completely and a strong electrolyte is defined as the oppposite how come the answer is B and









![BS005] 3M Sodium Acetate, pH 5.2 | Biosolution BS005] 3M Sodium Acetate, pH 5.2 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2016/02/BS005-Sodium-Acetate-Solution.jpg)
